|
Are we just “placeholders” for
the real inventors of proportional dosing?
Before we get to all the minutiae about our patents, let’s have a look at our big picture. We were not the first
to invent our method of dosing by weight. The credit for that goes to Janssen Pharmaceutica. We have hard evidence that they
were doing it well before us. I came up with it in September 1988. By that time in Switzerland they were already writing drafts
that included it for their label for Prepulsid. Even earlier, in March of that year, they were testing the device in Italy.
I think it was invented in the Belgium headquarters.
Now, given that they have beaten us, what are our options? We can’t claim inventorship, but we still beat them
patent-wise, at least as far as we know. By luck we happened to get our paper work in to the patent office (a quick and easy
process) before they got regulatory approval to begin sales (a time consuming and difficult process). This thing is so close
we may some day find that we do not have valid patent rights.
What should we do next? Yall might want to shoot me, but here is my sense of what is fair. In our articles and such,
we give Janssen full credit for pioneering dosing by weight. (This much we already do, including in our patent application.)
As far as the patent goes, we become co-owners. (Joint owners can both use the patent, license it to others, etc. All revenues
are split 50-50.). If they don’t want to own jointly with us, we do it unilaterally, giving them both a free license
and their share of licensing income. If they don’t take the money, we give it to a charity of our choice in their name.
(A likely charity would be one that was supporting clinical trials of dosing by weight.)
Patents and licensing
OptiDose - US patent progress. It has been more than a decade since I started (9/88) trying to get a patent
on our simple little medicine dropper with a body weight scale. The most recent news is not good. A few months ago I got an
“Office Action” (OA), which is how the patent office tells you all the things wrong with your application. Mary’s
opinion sums it up: “They don’t want to give you the patent”.
There were 3 objections. All seemed like a time warp – we have dealt with these before. First there was that
business about the Italian dropper. (I’m sure you remember it from out report 4 years ago. Sure.) Janssen tested their
new dropper in Italy well before us. In the last report many of you read the law, which not-very-clearly said that it didn’t
count if the public use was in a foreign country. We all agreed, and even the patent office manual says the same thing. Surely
we are OK on this one.
Second was the old label from Switzerland. It has a date of Sept 1988 on it, which would be killer IF it were a publication.
But last time we gave them the explicit dates of approval for publication (May 23, 1989), and actual publication (Nov 89),
both well after us (Nov 1988, the first stuff I sent to patent office).
Third was obviousness. This one is at least arguable. I will spare you all the details of this one, because it will
be just me saying I’m right and they’re wrong. I do think we’re fine on it. The pisser is that they didn’t
even mention the worst “obviousness” evidence, which we found in the spring.
In April my nephew Ben O found the most killer prior art to date. It was a de-worming medicine (Equimectrin) that had
a body weight scale right on the syringe. A little digging by Jeannie M at the maker found its predecessor had the same thing
in 1984. ARRGH!
Actually, I was kind of relieved that we had finally found that last coffin nail. I had a great summer in Hawaii, and
didn’t think twice about it. But, when fall came around I decided to look at it more seriously. Jeannie sent me a sample,
and, as it turns out we are probably still alive after all. (Great, now I can look forward to another decade of spending $500
a year to be abused by the patent office.)
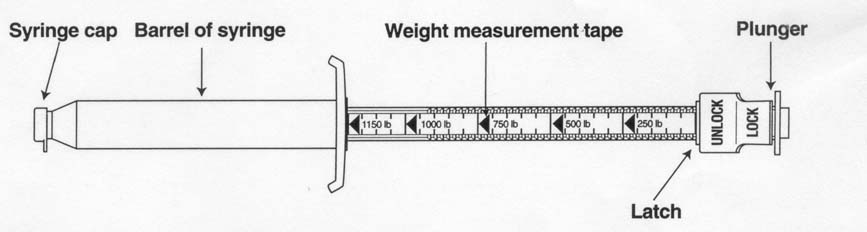
Their device measures the drug as it goes out. Theirs comes already full (it is a gel), and the scale goes backwards
relative to ours. Ours measures the dose as it is drawn up. It is a small difference, but it is physical, specific, and makes
theirs not work like ours. You could not use theirs to measure a daily dose by body weight (without doing math each day).
(So we are fine in the sense of it not being the same thing, before us. But someday I’ll have to argue that it would
not have been obvious to flip the scale around so that it would work.)
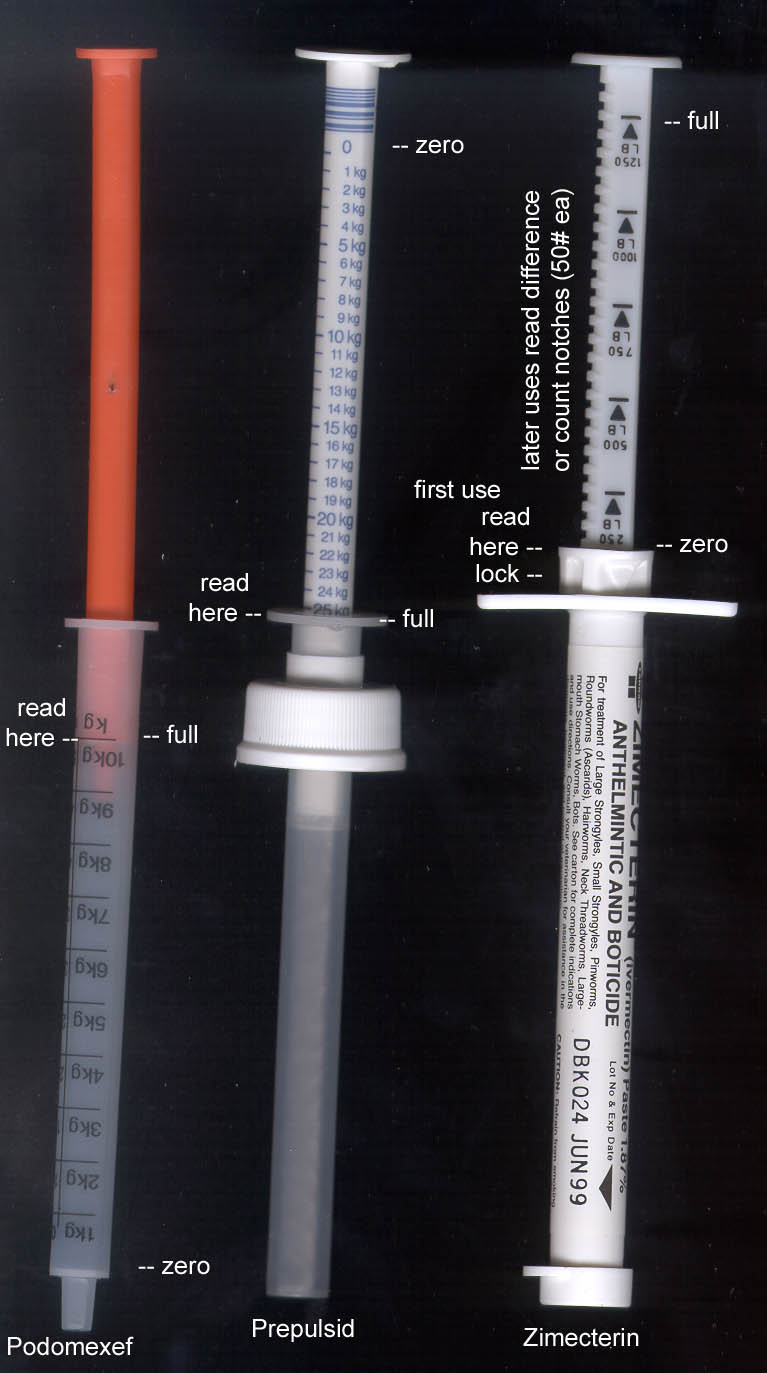
The two on the left are “our” type (from European products listed on the cover).
To get a dose by weight you just fill the syringe up to the body weight of the child. On the right is brand X’s syringe
design. It comes full, and you set the little latch thing to the body weight and then empty it to where the latch stops the
plunger. The next dose you have to do a little math to get the number to stop on next time. The cool thing about this design
is it comes ready-to-use, usually for one horse, one use, which is very convenient. But for us, it is quite different and
more complicated, which will help us at the patent office.
Flub-ups and minor patent issues
From earlier, here’s a little sequence that should raise doubts about my competence to manage our patents, and
about how long it will be until the patent office does anything:
1/16/97 new application turned in (this is CIP 5 on our OptiDose)
4/3/97 got notice that I paid fees wrong, owe another $195
4/18/97 I paid the fees while packing to move to Iowa
(Something apparently happened to this letter. I made at least two mistakes.
I did not put a post card in with it (which is your way of getting notice that they got something). And I didn’t notice
the check did not clear, partly because I set aside my Hawaii account.)
5/29/98 got notice of abandonment because I hadn’t paid
6/18/98 I paid, begged for mercy and “revival” on “unavoidable”
basis
3/5/99 Nix on revival because I couldn’t prove I mailed the check,
and because a reasonable man would have balanced his checkbook.
3/21/99 I begged for revival on “unintentional” grounds, which
means we lose all of the delay (=22 months) off the end of our patent term and have to pay another $660.
9-7-99 Petition granted. That put
us back in line, in the back. Next action is the OA above, a year later (9-25-00). God knows I’m sorry for losing that
check and for not balancing my checkbook.
Here’s a little older news. In the interim (on 10/17/97) I met with our examiner. At that time he sort of said
we’re OK on “obviousness”.
Another issue, or non-issue, is how long can we haggle over this patent. Some of you may have read that the US law
has changed from the old “haggle forever; patent lasts 17 years from issue” to the new “the clock starts
ticking at filing, and you have 20 years no matter when it gets issued”. We are still under the old rule, thank goodness,
since we filed way before June 7, 1995. However, we did lose 22 months (above), so our patent will last about 15 years.
OptiDose - Swiss licensing progress.
(Swiss Pat # CH 688 177 A5, granted June 13, 1997, term 20 yrs, very generous claims – any “body characteristic”
scale on any dosing device) Heinz and Julia B have done just about everything
that has been done on our Swiss patent. It has been a long battle. On April 4, 2000 I gave Heinz and Julia a 50% interest
in the patent. We (Prophy) and they are now joint owners.
Joint ownership means either of us may spend money on it. If there are revenues we each get half, even without
further work or investment. I did not ask them to agree to do anything else in the future. They are free to “retire”
and still get their half of whatever happens in Switzerland. On the income side, I may well have given half of the only money
the company will ever see. I think it’s fair, and in all of our best interests. (You can bitch on the ballot if you
think I’m an idiot.)
Things got a little bit exciting in the fall of 1999. Heinz was organizing a big postgraduate education program for
Swiss pediatricians. Abbott sponsored it. (Abbott makes the antibiotic, Klaciped, which is one of the 4 products in Switzerland
that use "our" method of dosing by weight.) He got to know the product manager, who eventually contacted the company’s
lawyers in the USA, who eventually asked for terms for a license!
Heinz and Abbott came up with a fairly innovative kind of license; to make it a one shot deal. They pay X amount now,
and the license is good for the life of the patent. (We made our first offer for 75,000 Swiss francs [about $50,000]. The
patent is good until the year 2012. If any of you want to review our offer, give me a holler. It is short and sweet and easy
to e-mail.)
Being asked for a license is a milestone for us, but that is as far as we have gotten so far. Things now seem a bit
bogged down since we haven’t heard back. Heinz has some new ideas, but I’m not sure what they are. (They also
hit on Sankyo, the maker of one of the other users, Podomexef antibiotic. They never heard back.)
Heinz and Julia have done very well in the licensing department. This is not surprising for those of you who remember
their history. Without any training in patent law, it was Heinz who successfully negotiated our patent with their patent office.
They found the Swiss products that used it, including the one that looked like it might have come before us. In a fine show
of judgment, they stopped me from giving our patent application to Janssen. (I sent Heinz and Julia the signed documentation
to use to get Janssen to give us their history.) Then they researched that issue themselves and found the actual date the
product went on sale, which was a later (and OK) date. Since then they have been contacting many of the pharmaceutical companies
in Switzerland. Sometimes they get a little busy with their practices, but by and large they have kept up with everything
as well or better than a regular professional licensing company could. We could not ask for anyone better.
OptiDose - South African licensing progress. There are no users
there that we know of, but Eric reports that there are plenty of potential products, including most of the ones on our list
of users in other countries. I am trying to get addresses, contacts, etc., but it is a lower priority. In South Africa they
just give you a patent as long as it goes through an attorney. So the initial patent must be worthless. I am hoping they have
some kind of “challenge” procedure where eventually you get an enforceable patent. (If you’re a net surfer,
try to find us the addresses / contacts we need in South Africa: Janssen, Essex [=Schering?], Upjohn, Abbott, Pfizer, and
Fisons.)
OptiDose – US licensing notes. We have found a company that
can make pharmaceutical grade samples for us at little or no cost. (For comparison, it cost us something like $5-10 grand
to make our first droppers.) All we need is two things. First we need the “blanks”, like the cups or droppers
the product is now using. Then we need to create the “artwork”, which can be as simple as a scale printed out
on a laser printer. So, other than the usual apathy, there is no reason why we couldn’t make a nice presentation to
the manager of almost any product. (We are also working on some new product designs that we may be able to test market ourselves.)
Click to see one of my most grandiose schemes, the Wal-Mart plan.
Click for another scheme, Pedi-sales.
However, a far more realistic way to go may be to target new liquid pharmaceuticals before they go through clinical
trials. That avoids the “don’t fix it if it ain’t broke” wisdom. (If you owned a successful product
like children’s Tylenol, would you tinker with it?) But if you had a new molecule you were trying to get to be accepted,
would you test it with an elegant new dispenser? This “new molecule” strategy takes a fair amount of net searching
to find the “product pipeline” of each company, then figure out which ones are likely to be pediatric, and liquid,
and soon to be tested. Anybody want to help?
It is a minor point, but we may be “losing” two products that Janssen has been using their dosifier on
in various countries. Both Prepulsid and Hismanal are running into safety problems. I think they are being pulled from the
US market. (Ironically, some of the problems are from overdosing in the US, where they do not use the dosifier.) Anyway, these
products would have been good candidates for dosing by weight in the US, but now it is unlikely.
Osteo - US patent progress. This invention is a “new use”
of fluoride, taking it in early pregnancy to reduce birth defects. From the outset the fundamental problem has been lack of
a good clinical trial to prove it. But patents do not require proof.
This last fall I re-wrote the whole thing for the 6th time (CIP 6, 11/14/00). I hope I got rid of some lingering
language problems. Mainly I had to take out phrases that say I’m not sure it will work. There were also some squabbles
about how you say just who’s who. (If you are taking this before you are pregnant, to prevent birth defects in your
child who is not even conceived, who is getting the benefit? I got to dream up phrases like “…preventing heart
defects in the offspring of women of childbearing potential…”)
Earlier I met with our examiner (10/17/97) and he gave me a copy of a patent that had been allowed with claims to reduce
birth defects. (By Geber, who used a corn byproduct.) He suggested I model my claims after Geber’s. Next (12/5/97) we
got an office action on this application. This is the one on using fluoride to prevent birth defects, and the big problem
has always been “usefulness”. (One of the big three requirements for a patent: new, useful, and non-obvious.)
The basic beef (I think, they never really tell you squat as to their reasoning) had been that if the new use of fluoride
was not proven well enough to get FDA labeling, it could not be sold and was therefore not useful to anyone. (Some of you
had signed oaths long ago that it was useful to you. These might have helped.) On this last rejection, the examiner did not
cite the “usefulness” part of the law (101), so, wowzers, we are making progress. There have been quite a few
great studies that have come out showing fluoride deficiency symptoms in various birth defects, and they were probably the
biggest help. [Note to you would-be inventors. Notice the squeeze. If I had waited until now to turn in the application, I
would be fighting on obviousness.]
On our next office action (9/5/98) our sole rejection was still just a language issue (the “112’ part of
the law) with no further trouble from that pesky usefulness. I turned in a brand new application (12/14/98, CIP #5) with all
the language changes the examiner had suggested earlier. But the language problems continued. I went through a few rounds
(2-25-00, 7-24-00 office actions) on the 5th application.
Osteo – US licensing progress. Long ago I had discussed the
osteo theory with a small pharmaceutical company that made a prenatal vitamin with fluoride. A few months ago my contact there
called and wanted a copy of my most recent write up, as they are designing a new prenatal vitamin for early pregnancy. Don’t
hold your breath.
Osteo - Canadian patent died. I had given this one up for dead
a long time ago, but it officially died June 16, 1998
Trademark issues. That guy who offered $5k for our OptiDose mark
in the summer of ’97 faded away after we turned his offer down. I think it was some kind of scam. Then, on September
15, we got an office action that some other company (Schering) wants to register the same mark. Could they be thinking about
dosing by weight? (I don’t know, but Schering was one of the 4 companies doing it in Switzerland, with Cedax®. We have
proposed to their US guys to try it with their Claritin®.) But at any rate, to the trademark office we had to prove when we
first started using the trademark in actual commerce. We were lucky once again. Those droppers we had made as prototypes (back
in Aug ‘93) have been used for years now. I think I have “sold”
a few hundred of them.
Eventually (10/23/98) the trademark office said we were OK (that they were publishing our mark, and if no one else
objected, we would get it). I told Schering that we “would be glad to give you a license for not much more than having
our name mentioned on your label”. They responded that they had decided “a couple of years ago” not to pursue
the OptiDose mark, and that they had no interest in licensing it. Oh, well. Anyway, we got our registration (# 2,223,608 on
Feb 16, 1999) so add that ®.
Other users. If you search something like Yahoo you’ll find that the Rohm & Haas company uses Optidose for
a water quality tracer. That use is far enough away that it will not cause confusion with our pharmaceutical market, so is
OK. There are also two ~computer programs that calculate dosage that are using the mark. We’re in discussions.
Trademark details:
OPTIDOSE
Registered Feb 16, 1999
Reg. No. 2,223,608
International Class 010:droppers, syringes, cylinders, and
cups for use in connection with medicinal preparations.
Date first used in commerce 03/11/1995
Serial # 75/249408
Published in Official Gazette Nov 24, 1998
The Schering application that conflicted was serial # 74-687435.
Other news
Brand new book by the Drs. Glenn (12-00). This is an absolute riot to read. It has lots of helpful information,
of course, but the stories are the most fun. I am promoting the book (if for no other reason it mentions me!). If you want
a copy I can get you one if you’ll write a letter to the editor, etc. with your opinion of it. Let me know. If you’d
like to see it (or even buy it like a normal person) you can just go to Amazon.com and type in “prefect teeth”.
The book is How to have children with perfect teeth, Frances B Glenn DDS and William
Darby Glenn III, MD, 2000.
If you want to see a sample of Darby Glenn ripping into the dental lobby, ask me for a copy of the letter to Slavkin.
Frances just published a biting letter in JADA Dec 2000; 131:1674-76.
Historical note - our first entry into the medical literature. It
is only a sentence or two, but the Drs. Glenn mentioned our dose by weight method in their 1997 article: Prenatal fluoride for growth and development: part X. ASDC Journal
of Dentistry for Children Sept-Oct 1997; 64(5):317-321. They also worked in some of the “Osteo” citations,
and did an excellent job of jabbing at the dental lobby for withholding prenatal fluoride. (The main point of the paper is
showing color glossy pictures of some of the cells that make teeth, looking far more beautifully developed with the addition
of prenatal fluoride.) Reprints available.
Our first “fert” success. It is just a glimmer of success,
really. Jan R had been going through infertility hassles for a lifetime or two. She happened to start her fluoride supplement
about a week before a procedure called an “IUI”. The procedure worked. (Others had failed, which is common, and
she also took a drug “Lupron®” before this one.) Anyway, this is nothing as far as showing fluoride can help with
infertility, but it is a giant success for Jan and Brian and young Jennifer. Also Barbara T had been reading some of my fluoride
stuff during her own infertility struggles, and now is the mom of twins. I don’t know if she actually took any of the
advice. I abandoned that patent application long ago anyway, but I still hope it works.
Our first internet sales site. Nothing to write home about, but we have made OptiDose droppers available for
sale over the internet. Our own site is http://homepages.infoseek.com/~optidose
We also tried a listing on the eBay auction but didn’t get any good hits.
New idiotic inventions that will probably be dropped. These are
my best ones that I deemed worthy of the first baby step in patenting. (If you think these are bad, you should see my rejects.)
I just turn them in as “disclosures”, which only costs $10 and gets you a good date. It also gets you to at least
sketch out the idea. (I highly recommend this painless process to would-be inventors.) (And then try to bail out before the
next step, about $500 for a real patent application.)
Carrier: This is a political ploy. The law against prenatal fluoride only
applies to listed ingredients. What I want to patent is using fluoride as a carrier as a way around the law. So the drug label
would say:
Calcium (as 2.05 mg calcium fluoride)....................1.05 mg
As opposed to the normal way if prenatal fluoride became legal:
Fluoride (as 2.05 mg calcium fluoride).......................1 mg
With this little tweak in wording doctors could still see the fluoride was there, but as a carrier it would not break
the law. (And this patent would have a weird positive effect on us. This patent would only be valuable if PNF stayed illegal,
but we are so warped business-wise that killing our own success would give us fuel to keep working to legalize it. My alma
mater will probably rescind my master’s in finance for this.)
MeasureMouth: This is just
a little gizmo to measure the shape of the roof of the mouth. What I am hoping is that we can someday prove that fluoride
in early pregnancy can prevent “vaulted” mouths, and that we can associate these vaulted mouths with various other
problems. We need a good measuring system to do it. The only way it could move beyond a little research tool is if it turned
out to be a good diagnostic indicator of harder-to-detect problems (like congenital deafness), which I think it will.
Lic - DBW product candidates 1-30-03
Prednisone and TAMIFLU
A candidate for DBW:
At the University of Rochester, patients have been maintained
on daily prednisone for over a decade now. The target dose is 0.75 mg/kg/d. In case of side effects, mainly
weight gain, the dose reduced to 0.5 mg/kg/d or even to 0.25 mg/kg/d. Over the decade of follow-up, some children have come
off medication. In each case we have noticed a functional decline within 6 months of the taper. The lost abilities are not
regained even when the children have been restarted on medication.
Brands: (Deltasone, Liquid Pred, Metocorten,
Orasone, Panasol, Prednicen-M, Sterapred)
Deltasone = Pharmacia
& Upjohn
Another candidate: Roche
Laboratories Inc., 340 Kingsland Street, Nutley, New Jersey 07110-1199 http://www.rocheusa.com/products/tamiflu/pi.html#DOSAGE%20AND%20ADMINISTRATION
Pediatric Patients: The recommended oral dose
of TAMIFLU oral suspension for pediatric patients 1 year and older or adult patients who cannot swallow a capsule is:
|
Body Weight in kg |
Body Weight in lbs |
Recommended Dose
for 5 Days |
Number of Bottles
Needed to Obtain the
Recommended Dose |
|
<15 kg |
<33 lbs |
30 mg twice daily |
1 |
|
>15 kg to 23 kg |
>33 lbs to 51 lbs |
45 mg twice daily |
2 |
|
>23 kg to 40 kg |
>51 lbs to 88 lbs |
60 mg twice daily |
2 |
|
>40 kg |
>88 lbs |
75 mg twice daily |
3 |
An oral dosing dispenser with 30 mg, 45 mg, and 60 mg graduations
is provided with the oral suspension; the 75 mg dose can be measured using a combination of 30 mg and 45 mg. It is recommended
that patients use this dispenser. In the event that the dispenser provided is lost or damaged, another dosing syringe or other
device may be used to deliver the following volumes: 2.5 mL (1/2 tsp) for children <15 kg, 3.8 mL (3/4 tsp) for
>15 to 23 kg, 5.0 mL (1 tsp) for >23 to 40 kg, and 6.2 mL (1 1/4 tsp) for >40 kg.
On to the next page (next a series of 3 licensing visions - first is owner's strategy)
|